Viroids are sub-viral agents, which exist as small infectious particles. They are somewhat similar to viruses but possesses some unique properties in their evolutionary origin, morphology and functions.
In 1917, Diener was the first scientist who discovered and termed the non-bacterial infectious plant pathogens as “Viroids”.
Potato spindle tuber viroids (PSTV) was the first viroid discovered by T.O. Diener and W.B. Raymer in 1967. PSTV caused massive damage to potatoes. Its discovery led to the existence of some infectious entities smaller than viruses.
Content: Viroids
Meaning of Viroids
Viroids refer to the small, non-cellular sub-viral agents, which exist as obligate intracellular parasites like viruses, but differs in property by lacking a protein coat. They are approximately 200-400 nucleotides long.
Viroid primarily infects, replicates and induces serious disease in higher plants. The pathogenicity of viroid differs within the distinct host species. Viroids cause latent infection, and they may also induce chronic infections in their host plants.
Properties
- Viroids are the smallest infectious agents, which only consist of tiny circular RNA.
- Its RNA is covalently closed and not coated by protein subunits.
- They are similar to small nuclear RNA, which only comprises a single stranded circular RNA molecule.
- A plant pathologist, Theodor Diener discovered viroids in the year 1971. Till date, the mechanism of host infection by viroids is not clear.
- Sometimes, viroids show severe damages in one plant without showing any symptoms in other plants of the same species.
- It does not encode proteins.
- The nucleotide sequencing of viroids is the same as those observed in transposons and retroviruses.
- Viroids are acellular particles, which appear smaller in comparison to viruses.
- They are considered as the molecular fossils of RNA dominated by DNA and protein.
- Like viruses, viroids also go through the cell to cell movement via plasmodesmata and systemic transport via the phloem.
- The viroid’s replication cycle follows the same pathway of viruses, as they also function as the obligate intracellular parasites.
- Its replication requires RNA polymerase II to synthesize messenger RNA from DNA, by using viroid RNA as a template.
- They can induce symptoms in higher plants via a mechanism known as “RNA silencing”.
Classification
Viroids are classified into two families, depending upon the site of replication.
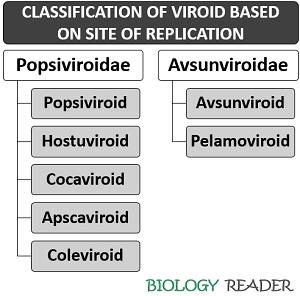
Popsiviroidae
The members belong to this family replicate inside the plant cell nucleus (nuclear viroids) and formerly called group-B viroids.
- Pospiviroid: It a genus that includes a type species (Potato spindle tuber viroid) with a genome size of 356 to 375 nt.
- Hostuviroid: It a genus that includes a type species (Hop stunt viroid) with a genome size of 295 to 303 nt.
- Cocadviroid: It a genus that includes a type species (Coconut cadangcadang viroid) with a genome size of 246 to 301 nt.
- Apscaviroid: It a genus that includes a type species (Apple scar skin viroid) with a genome size of 306 to 369 nt.
- Coleviroid: It a genus that includes a type species (Co/ens blumei viroid) with a genome size of 248 to 361 nt.
Avsunviroidae
The members belong to this family replicate inside the chloroplast (chloroplastic viroids) and formerly called group-A viroids.
- Avsunviroid: It a genus that includes a type species (Avocado sun-blotch viroid) with a genome size of 246 to 250 nt.
- Pelamoviroid: It is a genus that includes a type species (Peach latent mosaic viroid) with a genome size of 337 to 399 nt.
Thus, the site of replication differs within the type of plant species. However, the replication mechanism follows the same strategy of three steps RNA-based replication. Its replication cycle is dependent upon the following three enzymatic activities:
- Host DNA-dependent RNA polymerases
- Processing enzymes
- RNA ligase
Distinction Between Viruses and Viroids
- Viruses are nucleoprotein particles, while viroids are infectious RNA particles.
- Unlike viruses, viroids appear small and have a low molecular weight.
- Viroid only contains an RNA genome, whereas viruses may contain DNA or RNA. The infectious RNA replicates autonomously (without a helper virus) in the susceptible host cells.
Viroid Genome
Its genome size has a low molecular weight (1.1-1.3X105 Da). It can be characterized by the absence of protein coating surrounding the viral genome. Viroid’s genome lacks an AUG codon that mediates protein synthesis.
Viroid comprises a small fragment of RNA molecule that generally exists as a naked and circularized RNA. The genomic RNA consists of 250-370 nucleotides. The nucleotides in the RNA genome are usually paired, forming a dsRNA via intramolecular complementary regions.
Therefore, viroids resemble a rod-like structure, where the dsRNA appears as a closed, folded and 3D structure.
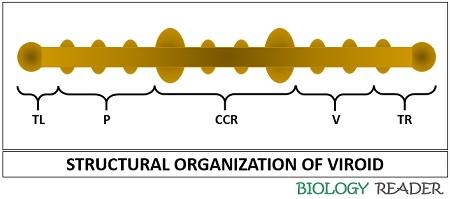
Viroid genome generally comprises five domains:
- Pathogenicity domain (P)
- Left terminal domain (TL)
- Central conserved domain (CCR)
- Variable domain (V)
- Rigid terminal domain (TR)
The folded regions of the viroid help in protecting the RNA against the action of cellular enzymes.
Replication of Viroids
The replication of viroid in higher plants occurs via three enzyme based-RNA rolling circle mechanism. Symmetric replication occurs in Avsunviroids, whereas asymmetric replication occurs in Pospiviroids.
RNA polymerase, an RNase and an RNA ligase are the enzymes needed for viroid replication. Symmetric and asymmetric replication are the two replicative mechanisms of viroid.
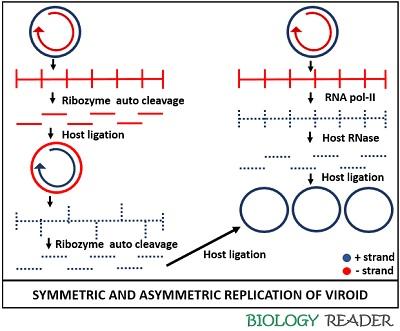
Asymmetric Replication
Members of the Popsiviroidae family undergo asymmetric replication, for which they primarily require a host cell enzyme (RNA polymerase II). The RNA polymerase II creates a nick in the cellular DNA by forming both positive and negative strands.
The positive circular strand will function as a template in producing new RNA via a rolling circle mechanism. Then, it initiates the synthesis of mRNA from the cellular DNA, and make a large linear multimeric (-) strand by using a viroid circular (+) RNA strand.
After that, Pospiviroids form (+) sense RNA from this long linear molecule via an asymmetric replication pathway. The (+) RNA strand of viroid is sensitive to the RNase activity of the host cell.
Thus, the RNase enzyme cleaves the (+) RNA strand of viroid into unit viroid lengths. Then, the unit fragments of the (+) RNA strand molecule undergo ligation to form the (+) circular RNA.
Symmetric Replication
Avsunviroids lack a central conserved region and possess a ribozyme activity instead of RNA polymerase. Hence, the large multimeric (-) RNA strand is self-cleaved by the associated ribozyme activity.
Therefore, avsunviroids possess the property of auto cleavage. Then, the replication intermediates ligate into a (-) circularized RNA.
After that, a second rolling circle event takes place that makes a long linear (+) RNA strand. The ribozyme activity again cleaves the strand and eventually, the fragments of viroid RNA ligate into a (+) circular RNA.
Genome replication occurs in two possible ways, namely RNA and DNA directed replication. RNA-directed replication is one of the mechanisms, where the RNA polymerase synthesizes RNA molecules directed by the RNA itself.
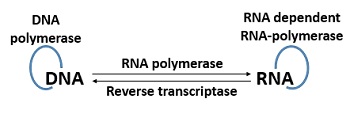
DNA-directed replication is another mechanism, where the viroid RNA forms after the transcription of host cell DNA, complementary to the viroid RNA. The viroid RNA produces new DNA in the infected cell via association of reverse transcriptase enzyme.
The reverse transcriptase enzyme functions as an RNA directed DNA polymerase, which produces viroid RNA by using the infected DNA as a template.
After the study of these two replication modes, two conclusions have been made that the viroids can replicate via direct RNA to RNA copying, and the host cell machinery possibly promotes the replication of viroid RNA.